Recent years have witnessed significant efforts toward the development of mini-organs known as organoids in the culture dish. Basically, organoids are tiny, self-organized, 3-D tissue cultures that are derived from stem cells. Organoids contain various cell variants and complex microarchitectures present in human organs like kidney, liver, intestine, and even the brain. They are designed to replicate the complexity of an organ or to express selected functions of it like producing only certain types of cells.
The challenge
Most of the mini organs grown in vitro lack vasculature necessary to provide oxygen and nutrients, remove metabolic waste, and facilitate communication between different cell types that results in their maturation into truly functional tissue building blocks.
In the case of kidney organoids, this limitation obstructs researchers from imitating key kidney functions in vitro, including blood filtration, reabsorption, and urine production. Creating sturdy vascularized kidney organoids could enable researchers in better modeling of kidney diseases, enhance renal drug toxicity testing and, ultimately, lead to new building blocks for renal replacement therapies.
What is new
Now, a collaborative team of researchers have developed a new method of organoid development. Researchers subjected the stem cell-derived organoids to fluidic shear strain, they found significantly enlarge organoid-derived vascular networks and improved maturation of kidney compartments in comparison to previous static culture methods. The work is published in Nature Methods.
“While our organoids and those generated in other laboratories contained large numbers of well-organized nephrons and primitive blood vessels, they still lacked pervasive vascular compartments with perfusable lumens,” said co-corresponding author Morizane, M.D., Ph.D., Assistant Professor at Brigham and Women’s Hospital and Harvard Medical School (HMS), and a member of the Harvard Stem Cell Institute.
Researchers across the globe used to mature kidney organoids by implanting them into animals where they can associate to the host’s vasculature in vivo.
“For the first time, our study demonstrates that by exposing growing organoids to fluid flow, a mechanical cue known to play an important role for tissue development in the body, we can greatly enhance their vascularization and maturation in vitro,” said Morizane.
What they did
The research team used expertise from the Lewis lab that has established procedures to create vascularized human tissues, using 3D bioprinting that can be perfused and sustained for a long time span. Based on these findings, the researchers hypothesized that fluid flow could also promote the formation of blood vessels from precursor endothelial cells found in growing kidney organoids.
“We determined the right combination of underlying extracellular matrix, media additives, and fluidic shear stress under which human stem-cell derived organoids would flourish when grown in our 3D-printed millifluidic chips,” said Kimberly Homan, Ph.D., who is a first author on the study and working as Research Associate in Lewis’ group at the Wyss Institute and SEAS.
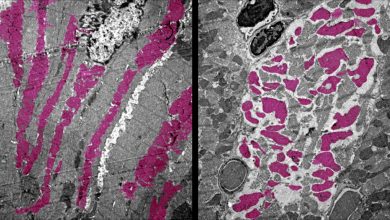
The vessels growing on the 3D-printed chips form an intermeshed network with open lumens, which can be perfused with fluids as confirmed by directly imaging fluorescent beads moving freely through them.

“The vascular networks form close to the epithelial structures that build the glomerular and tubular compartments, and in turn promote epithelial maturation. This integrated process works really like a two-way street”, added Navin Gupta a Clinical Research Fellow working on Morizane’s team at the Brigham
Why it matters
Latest findings can open new ways for precisely testing drug toxicity in vitro in differentiated nephron compartments and modeling kidney diseases, like polycystic kidney disease, that affect specific structures. It may also pave the way to vascularize other types of organoids, such as the liver organoids.
“This study is a great example of the importance of mechanobiology and the potential power of the Wyss Institute’s 3D Organ Engineering Initiative. It provides an important cornerstone for many efforts that aim to create functional human tissues de novo for research, pharmaceutical and tissue regenerative applications,” said Wyss Institute Founding Director Donald Ingber.
The study was funded by the National Institutes of Health, Harvard’s Wyss Institute for Biologically Inspired Engineering, and the Harvard Stem Cell Institute.