Cell signaling, which is often referred to as signal transduction or transmembrane signaling, is the process by which cell communicates with the environment and respond to external cues that they sense. In order to respond to changes in their immediate environment, cells must be able to receive and process signals that originate outside their borders. But cells aren’t just targets, they also send out messages to other cells, both near and far.
Most cell signals are chemical in nature, for example, prokaryotic organisms have sensors that detect nutrients and help them navigate towards food sources. In multicellular organisms, growth factors, hormones, neurotransmitters and extracellular matrix components are some of the many types of chemical signals cell use. Cells have proteins called receptors that bind to the signalling molecules and initiate a physiological response. Different receptors are specific for different molecules, e.g. insulin receptors find insulin, dopamine receptors find dopamine, etc. Needless to say, any perturbation of this biochemical signaling can be extremely detrimental to the cells, even leading to cancer in some cases. If these Pathways are studied properly, they can give us insights to treat cancer.
One such research was conducted in Tokyo University of Science by professor Assoc Yuuki Obata and his team.
The KIT gene provides instructions for making a member of a protein family called receptor tyrosine kinases. Receptor tyrosine kinases transmit signals from the cell surface into the cell, through a process called signal transduction. The signaling Pathways stimulated by the KIT protein control many important cellular processes such as cell growth and division (proliferation), survival and movement.
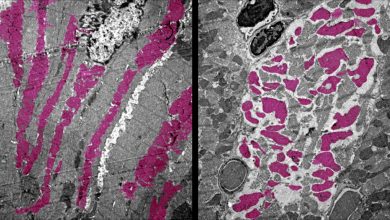
The KIT protein signaling is very important for the development and function of certain types including reproductive cells (germ cells), early blood cells (hematopoietic stem cells), white blood cells called mast cells, cells in the gastrointestinal tract called interstitial cells of Cajal (ICCs), and cells called melanocytes. Active mutation of these proteins has been identified in several cancers such as gastrointestinal stromal tumor (GIST), Mast cell leukemia (MCL), and acute myeloid leukemia (AML). In MCL the mutations D816V (human) and D814Y (mouse) are frequently found; here the mutated KIT protein is mislocalized in a cellular compartment, called endolysosome (EL). In GIST, mutated KIT accumulates inside and conduct cancer-specific signaling from the Golgi. Active KIT mutations have been found in about 10% of core-binding factors (CBF- AML) patients.
However, it remains unclear whether KIT transducers signals from intracellular compartments in (AML). The research from Japan aims to answer this question by using a newly synthesized compound called M-COPA, that targets intracellular transport and can be used to combat cancer.
Prof. Shiina says, “We wanted to investigate the anticancer effect of the new anticancer drug lead compound M-COPA synthesized at our university against hematological cancers (leukemia, lymphoma, etc.).”
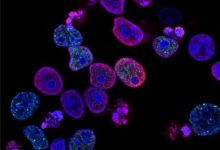
Leukemia cell lines, such as Kasumi-1 (KITN822K, AML), SKNO-1 (KITN822K, AML), and HMC-1.1 (KITV560G, MCL), were used by the researchers to explore how KIT transduces signals in these cells and for examining the single platform for the mutant using immunofluorescence microscopy and inhibition of intracellular trafficking. Next, In AML cell lines, KITN822Kaberrantly localizes to EL. After biosynthesis, KIT traffics to the cell surface via the Golgi and immediately migrates to EL through endocytosis in a manner dependent on its kinase activity.
However, results of phosphorylation imaging show that KIT is preferentially activated on the Golgi. Indeed, blockade of KITN822K migration to the Golgi with BFA/M-COPA inhibits the activation of KIT downstream molecules, such as AKT, ERK, and STAT5, indicating that KIT signaling occurs on the Golgi. Moreover, lipid rafts in the Golgi play a role in KIT signaling. Interestingly, KITV560G in HMC-1.1 migrates and activates downstream in a similar manner to KITN822K in Kasumi-1.
Prof. further concluded that “In AML, KITN822K mislocalizes to EL. Our findings, however, suggest that the mutant transduces phosphorylation signals on lipid rafts of the Golgi in leukemia cells. Unexpectedly, the KITV560G signal platform in MCL is similar to that of KITN822K in AML. These observations provide new insights into the pathogenic role of KIT mutants as well as that of other mutant molecules.”