A new research at The Scripps Research Institute has opened the chapter of the enigma that how memory T cells originate. Memory T cells protect against previously encountered pathogens, but their origins are unclear.
Memory T cells are a subset of infection- and cancer-fighting T cells. Memory T cells have become “experienced” by having encountered antigen during a prior infection, an encounter with cancer, or previous vaccination.
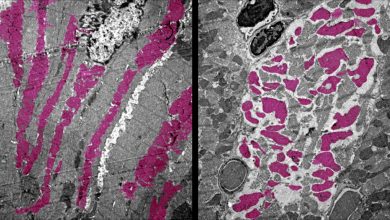
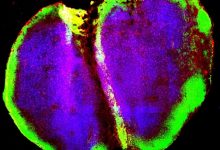
During their study, researchers found that there is a transcription factor protein which is directing dividing T cells to become memory T cells. These transcription factor protein are called Runx3.
T cell differentiation regulation by Runx3’s is essential because when our bodies fight off viruses and cancers and on response our T cells burst into action, the majority of the dividing cells tend to become effector cells. These effector cells are short-lived and do not persist once the infection resolves. Runx3 controls the t cell differentiation and put dividing T cells on track to become memory T cells which can live for decades.
Runx3 turns off another transcription factor controlling terminal effector cells proliferation to keep development of memory T cell on track.
“This finding provides molecular evidence that the programming of memory is established very rapidly, and that it’s kind of a push and pull to restrain the developing memory cells from differentiating into effector cells, which is a dead-end road,” says Pipkin.
This new discovery can help researchers to design drugs for enhancing the immune responses to vaccines, Pipkin says. Furthermore, this new insight has implications for chronic diseases such as cancer responding which T cells become tired and unable to rally an effective defense.
“There are instances such as chronic infection and tumors where the T cells differentiate in an aberrant way that shortens their lifespan and decreases their function,” Pipkin says. “Because our study found that Runx3 is one of the earliest players during an immune response, manipulating it might be an avenue to basically turn back the clock and reprogram dysfunctional T cells into a format that is conducive to them developing into genuine memory cells that are protective.”
In the light of the study, researchers want to determine if some type of therapeutic could rescue naturally occurring Runx3 deficiencies. They also want to identify the other players that cooperate with Runx3.