A new method has been developed to use old drugs to fight cancer
Cancer is a deadly disease of uncontrolled growth of cells in the body. According to WHO reports more than 3.7 million new cases are seen and 1.9 million dies of cancer each year.
Chemotherapy and Radiotherapy are used for the treatment of this disease. Recently developed drugs called kinase inhibitors are proving to be very effective in the treatment of cancer. They attack a family of receptors tyrosine kinases (RKTs), which protrude from cell surface like antennae, these RKTs activate cancerous activities in the cell.
A new method has been developed to use these old drugs to fight cancer. Researchers at Arizona State University ‘s Biodesign Institute developed a method to screen a broad range of kinases for a drug’s effectiveness. Ibrutinib which is inhibitors of Bruton’s tyrosine kinase (BTK) found in white blood cells, was approved by FDA in 2013 for treatment of leukemia.

A new research conducted by Dr. Joshua LaBaer who is Director at Biodesign Institute shows that ibrutinib can also target member of RTK family ERBB4 which block the sequence of events leading to cancer.
Results of the research show that ibrutinib act as an inhibitor of ERBB4 and limits the growth of cancer cells in the laboratory and reduce the size of the tumor in mice. The sensitivity of ERBB4 is similar to that of BTK.
This study provides a method for searching new targets of these old drugs like kinases inhibitors demonstrates the potential therapeutic utility of an existing pharmaceutical to selectively target ERBB4, in addition to its original target BTK.
LaBaer said, “We are particularly excited because it demonstrates that the proteins on these arrays are functionally active in a manner that reflects their natural activity and enables us to screen for their responses to selective drugs. This now provides a tool that would enable us to look for off-target responders to drugs or determine how specific acquired mutations might affect drug response.”
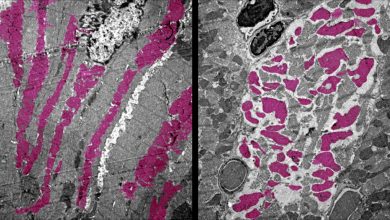
ERBB4 is a little-studied family of RTK, to explore its behavior researchers are utilizing a technique called protein microarray. In this technique, a microscopic surface is used on which many different proteins can be studied simultaneously, each protein is represented by a dot. Interaction of a protein with other proteins, their binding activities with DNA and RNA or with other factors found in blood and a broad range of pathogens can be studied.
The conventional method of printing proteins on the slide is an inefficient and laborious process as proteins are delicate three-dimensional structure, that must be folded in the right way to function properly. But both the method conventional printing protein and the new method of protein array are used in non-human systems, therefore, an elaborate protein purification steps are required, which makes it difficult to stabilize the structure of protein for a period of time.
A revolutionary technology known as NAPPA ( nucleic acid programmable protein array ) is used in place of printing slides or protein microarray. NAPPA prints circular DNA called plasmid, coding for required protein then synthesizes the target proteins just-in-time on the spot by adding human cell extract for more natural protein folding. NAPPA provides a highly efficient and functional way of protein expression and screening.
Dr. Femina Rauf lead author of the new study said, “You can express functional protein within one and a half hours. That’s the cool thing about it. We are using the human lysate system to express the proteins, so we expect to see well-folded, functional proteins. We’re especially excited because the RTKs are membrane proteins, which are ordinarily challenging to express but are still active here.”
Developing cancer-fighting drugs at economical cost takes decades, thus repurposing the use of the existing drugs becomes an attractive prospect.